Introduction
Photodynamic therapy is a method for local destruction of tissue or organisms by generating toxic oxygen and other reactive species using light absorbed by an administered or an endogenously generated photosensitiser.
It is a highly promising treatment for patients with cancer. More recently it has found increasing use as a method of therapy for non-cancerous illnesses. It depends on the exploitation of natural and vital reactions widespread in nature that have driven and preserved life on this planet. Following administration of a photosensitiser or its precursor there is an accumulation or retention in areas of cancer and disease relative to adjacent normal tissue. The photosensitiser is inactive until irradiated by light, following which cellular destruction occurs. The clear attraction of this method is the possibility of some targeting of the disease by drug and by the area irradiated.
Eukaryotic complex multi-cellular organisms have a high degree of metabolic specialisation with a requirement for oxygen and carbon species for oxidative phosphorylation. Yet the generation of reactive oxygen species is an initiator of apoptosis and cell death. Eukaryotic cells have therefore developed methods to resist oxidation. Evolutionary biologists believe that cells overcame this problem by the endosymbosis of mitochondria, a chloroplast like energy complex derived from cellular incorporation of primitive protobacteria. The mitochondria also contain natural photosensitisers called porphyrins, which are necessary for manufacture of the oxygen carrier haemoglobin and the energy transfer system involving the cytochromes.
 These are naturally generated or endogenous photosensitisers able to absorb light and generate toxic oxygen species under certain circumstances. Human cells have protective mechanisms against this toxic oxygen damage and cell death will only occur if a critical threshold of toxic oxygen species is reached and the protective mechanisms associated with the mitochondria are overwhelmed. This threshold effect is important since it can be exploited to allow preservation of normal tissues. Some unfortunate individuals are afflicted with a disease in which excessive photosensitisers are generated by in-born errors of metabolism.
These are naturally generated or endogenous photosensitisers able to absorb light and generate toxic oxygen species under certain circumstances. Human cells have protective mechanisms against this toxic oxygen damage and cell death will only occur if a critical threshold of toxic oxygen species is reached and the protective mechanisms associated with the mitochondria are overwhelmed. This threshold effect is important since it can be exploited to allow preservation of normal tissues. Some unfortunate individuals are afflicted with a disease in which excessive photosensitisers are generated by in-born errors of metabolism.
These disorders are called porphyrias and lead to excessive accumulation of porphyrin photosensitisers, which when activated by light in the skin result in profound tissue damage overcoming the cells natural defences. These patients are exquisitely sensitive to light; this is most evident in patients with acute intermittent porphyria who are deficient of porphobilinogen deaminase.
This inherited disorder was highly prevalent in central Europe. The afflicted individuals were exquisitely sensitive to light on the skin and developed excess body hair. In addition, the patients could have red teeth, be unable to venture out during daylight, and have mental
disorders. The discovery that an administered substance could render an organism sensitive to light (photosensitivity) is attributed to Oscar Raab working in Munich. In the winter of 1887–98, Professor Herman von Tappeiner set his student Raab to study the toxicity of aniline dyes on paramecia. Raab recognised that the time to kill was related to the intensity of light in the laboratory.
The most dramatic investigation of photosensitization was by Meyer-Betz who injected himself with a porphyrin compound (haematoporphyrin) and observed the effects of sunlight on his skin.
He published a series of photographs of himself suffering from severe photosensitivity with gross facial oedema and erthyema. He remained sensitive to sunlight for over 2 months. examined many other substances including chlorophyll and called the phenomenon “photodynamic action/photodynamische Erscheinung”.
He demonstrated that a photosensitiser, light and molecular oxygen were necessary. He also suggested that tumours could be treated and some early clinical results were reported in 1905 in combination with the dermatologist Jesionek. They applied eosin to skin tumours and exposed them to white light with some response. Subsequently, the parenteral administration of eosin by the French neurologist, J. Prime, as a treatment for epilepsy resulted in a light induced dermatitis in exposed areas of the skin.
In Berlin during the Second World War, Auler and Banzer demonstrated that photosensitisers tended to localise in tumour and malignant tissue. They injected animals with haematoporphyrin and showed increases of fluorescence in animal cancers.
Dougherty established the modern era working at the Division of Radiation Biology at Roswell Park Memorial Institute, Buffalo, USA. He reported that the systemically injected porphyrin (haematoporphyrin) when activated by red light caused complete eradication of transplanted experimental tumours. He also confirmed the preferential accumulation of the photosensitiser in malignant tissue.
Biology and photophysics of clinical photodynamic Therapy
As stated in the introduction, the destruction of abnormal and diseased tissue after generating or administering a photosensitiser with the direct application of light forms the basis of photodynamic therapy.
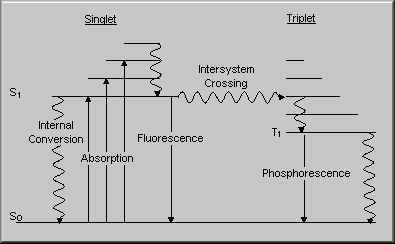
The requirements are a photosenitiser, light, oxygen and a substrate to act upon. Each photosensitiser has a specific action spectrum that is the wavelengths of light that are absorbed to produce an excited electronic state.
Photodynamic therapy and tissue destruction requires the excited singlet to undergo spin inversion (intersystem crossing) to the metastable triplet state. The triplet state has a longer lifetime and is generally the reactive state involved in photodynamic therapy. The most usual subsequent action is for the activated triplet photosensitiser to transfer energy to ground state oxygen (which is a triplet) to produce singlet oxygen. This molecule is highly reactive and cannot diffuse far before reacting with other molecules. Major biological targets are membranes that undergo rupture and the cells are destroyed. It has been recently demonstrated that most damage is to the membranes around the mitochondria and the lysosomes. These organelles liberate destructive proteins that induce subsequent cellular destruction.
Selective tumour destruction can be achieved if the photosensitiser is administered in low dosage, since the photosensitiser is photodegraded (in normal tissue) by light irradiation before a critical lethal threshold photodynamic dose is reached. However, tumours that selectively retain a higher concentration of photosensitser are destroyed because this threshold photodynamic dose is achieved and cell death is inevitable.
It is also noticeable that some normal tissues are remarkably resistant to photodynamic therapy. They appear to have a naturally higher photodynamic threshold. This is most apparent in the pancreas. The normal pancreatic acinar cell contains many mitochondria and is
very resistant to oxidative stress, the mitochondria being the ingested ‘chloroplast’. This appears essential since it produces such a cocktail of digestive enzymes and must resist auto digestion. Malignant pancreatic cells have fewer mitochondria and thus fewer toxic oxygen quenching molecules, and are therefore much more sensitive to photodynamic therapy. Selective necrosis of tumours with sparing of normal pancreatic tissue can be demonstrated in experimentally induced tumours.
The most commonly used method of photodynamic therapy is to administer a photosensitiser, intravenously, orally or by local application to an area of abnormality and allow retention and accumulation in the tissue for a period of time prior to irradiation with appropriate wavelength light, usually from a laser. These externally administered photosensitisers tend to accumulate in rapidly growing tissue, blood vessels and the supporting tissue that grows with malignant tumours. Parenteral administration either by injection or by mouth does produce a period of general photosensitivity and accumulation is in stromal supportive tissue rather directly within growing cells.
Cancer
Photodynamic therapy has attracted most interest as a method for the local eradication of cancer. The initial treatment of patients was of large areas of tumour that could not be treated by other means, or had failed conventional therapy following surgery, radiotherapy or chemotherapy. The treatment of advanced cancers is effective in palliation of some of the difficult symptoms associated with blockage of food or air passages.
Recently great interest has been shown in the treatment of biliary and pancreatic cancer. Bile duct or cholangiocarcinoma can be a relatively indolent tumour but treatment with surgery, radiotherapy and chemotherapy is very difficult.
Pilot studies have demonstrated that endoscopic Photofrin-PDT is also effective in the palliative treatment of hilar cholangiocarcinoma. A Phase III trial compared stenting plus PDT (n=20) with stenting alone (n=19) and showed a prolongation of survival by almost a year in stenting plus PDT group. The most recent study of eight patients, who underwent 1-5 treatments, showed that median survival from the date of the first PDT treatment was 276 days, whereas median survival times were 45 and 127 days for bismuth type III and IV tumors treated with stenting alone. The five-year follow-up data of 23 patients showed that median survival after treatment was 11.2 months for MO patients and 9.3 months for all patients. The 1-year, 2-year, 3-year, and 4-year survival rates were estimated to be 47%, 21%, 11%, and 5%, respectively, for MO patients and 39%, 17%, 9%, and 4%, respectively, for all patients. Preliminary results confirm that endoscopic illumination of the biliary tract is safe and effective for inoperable cholangiocarcinoma and can improve cholestasis, performance, and quality of life for an extended period. Since endoscopic PDT appears to be the first approach leading to an improvement in prognosis, it should be offered to patients with inoperable cholangiocarcinoma. Preliminary studies suggest that operative PDT might also improve survival for those patients undergoing surgical resection
Links
Medscape
Journal of Hepatology and Gastroenterology
Journal of Gastroenterology
Pubmed
Photodynamic therapy is a method for local destruction of tissue or organisms by generating toxic oxygen and other reactive species using light absorbed by an administered or an endogenously generated photosensitiser.
It is a highly promising treatment for patients with cancer. More recently it has found increasing use as a method of therapy for non-cancerous illnesses. It depends on the exploitation of natural and vital reactions widespread in nature that have driven and preserved life on this planet. Following administration of a photosensitiser or its precursor there is an accumulation or retention in areas of cancer and disease relative to adjacent normal tissue. The photosensitiser is inactive until irradiated by light, following which cellular destruction occurs. The clear attraction of this method is the possibility of some targeting of the disease by drug and by the area irradiated.
Eukaryotic complex multi-cellular organisms have a high degree of metabolic specialisation with a requirement for oxygen and carbon species for oxidative phosphorylation. Yet the generation of reactive oxygen species is an initiator of apoptosis and cell death. Eukaryotic cells have therefore developed methods to resist oxidation. Evolutionary biologists believe that cells overcame this problem by the endosymbosis of mitochondria, a chloroplast like energy complex derived from cellular incorporation of primitive protobacteria. The mitochondria also contain natural photosensitisers called porphyrins, which are necessary for manufacture of the oxygen carrier haemoglobin and the energy transfer system involving the cytochromes.
 These are naturally generated or endogenous photosensitisers able to absorb light and generate toxic oxygen species under certain circumstances. Human cells have protective mechanisms against this toxic oxygen damage and cell death will only occur if a critical threshold of toxic oxygen species is reached and the protective mechanisms associated with the mitochondria are overwhelmed. This threshold effect is important since it can be exploited to allow preservation of normal tissues. Some unfortunate individuals are afflicted with a disease in which excessive photosensitisers are generated by in-born errors of metabolism.
These are naturally generated or endogenous photosensitisers able to absorb light and generate toxic oxygen species under certain circumstances. Human cells have protective mechanisms against this toxic oxygen damage and cell death will only occur if a critical threshold of toxic oxygen species is reached and the protective mechanisms associated with the mitochondria are overwhelmed. This threshold effect is important since it can be exploited to allow preservation of normal tissues. Some unfortunate individuals are afflicted with a disease in which excessive photosensitisers are generated by in-born errors of metabolism.These disorders are called porphyrias and lead to excessive accumulation of porphyrin photosensitisers, which when activated by light in the skin result in profound tissue damage overcoming the cells natural defences. These patients are exquisitely sensitive to light; this is most evident in patients with acute intermittent porphyria who are deficient of porphobilinogen deaminase.
This inherited disorder was highly prevalent in central Europe. The afflicted individuals were exquisitely sensitive to light on the skin and developed excess body hair. In addition, the patients could have red teeth, be unable to venture out during daylight, and have mental
disorders. The discovery that an administered substance could render an organism sensitive to light (photosensitivity) is attributed to Oscar Raab working in Munich. In the winter of 1887–98, Professor Herman von Tappeiner set his student Raab to study the toxicity of aniline dyes on paramecia. Raab recognised that the time to kill was related to the intensity of light in the laboratory.
The most dramatic investigation of photosensitization was by Meyer-Betz who injected himself with a porphyrin compound (haematoporphyrin) and observed the effects of sunlight on his skin.
He published a series of photographs of himself suffering from severe photosensitivity with gross facial oedema and erthyema. He remained sensitive to sunlight for over 2 months. examined many other substances including chlorophyll and called the phenomenon “photodynamic action/photodynamische Erscheinung”.
He demonstrated that a photosensitiser, light and molecular oxygen were necessary. He also suggested that tumours could be treated and some early clinical results were reported in 1905 in combination with the dermatologist Jesionek. They applied eosin to skin tumours and exposed them to white light with some response. Subsequently, the parenteral administration of eosin by the French neurologist, J. Prime, as a treatment for epilepsy resulted in a light induced dermatitis in exposed areas of the skin.
In Berlin during the Second World War, Auler and Banzer demonstrated that photosensitisers tended to localise in tumour and malignant tissue. They injected animals with haematoporphyrin and showed increases of fluorescence in animal cancers.
Dougherty established the modern era working at the Division of Radiation Biology at Roswell Park Memorial Institute, Buffalo, USA. He reported that the systemically injected porphyrin (haematoporphyrin) when activated by red light caused complete eradication of transplanted experimental tumours. He also confirmed the preferential accumulation of the photosensitiser in malignant tissue.
Biology and photophysics of clinical photodynamic Therapy
As stated in the introduction, the destruction of abnormal and diseased tissue after generating or administering a photosensitiser with the direct application of light forms the basis of photodynamic therapy.
The requirements are a photosenitiser, light, oxygen and a substrate to act upon. Each photosensitiser has a specific action spectrum that is the wavelengths of light that are absorbed to produce an excited electronic state.
Photodynamic therapy and tissue destruction requires the excited singlet to undergo spin inversion (intersystem crossing) to the metastable triplet state. The triplet state has a longer lifetime and is generally the reactive state involved in photodynamic therapy. The most usual subsequent action is for the activated triplet photosensitiser to transfer energy to ground state oxygen (which is a triplet) to produce singlet oxygen. This molecule is highly reactive and cannot diffuse far before reacting with other molecules. Major biological targets are membranes that undergo rupture and the cells are destroyed. It has been recently demonstrated that most damage is to the membranes around the mitochondria and the lysosomes. These organelles liberate destructive proteins that induce subsequent cellular destruction.
Selective tumour destruction can be achieved if the photosensitiser is administered in low dosage, since the photosensitiser is photodegraded (in normal tissue) by light irradiation before a critical lethal threshold photodynamic dose is reached. However, tumours that selectively retain a higher concentration of photosensitser are destroyed because this threshold photodynamic dose is achieved and cell death is inevitable.
It is also noticeable that some normal tissues are remarkably resistant to photodynamic therapy. They appear to have a naturally higher photodynamic threshold. This is most apparent in the pancreas. The normal pancreatic acinar cell contains many mitochondria and is
very resistant to oxidative stress, the mitochondria being the ingested ‘chloroplast’. This appears essential since it produces such a cocktail of digestive enzymes and must resist auto digestion. Malignant pancreatic cells have fewer mitochondria and thus fewer toxic oxygen quenching molecules, and are therefore much more sensitive to photodynamic therapy. Selective necrosis of tumours with sparing of normal pancreatic tissue can be demonstrated in experimentally induced tumours.
The most commonly used method of photodynamic therapy is to administer a photosensitiser, intravenously, orally or by local application to an area of abnormality and allow retention and accumulation in the tissue for a period of time prior to irradiation with appropriate wavelength light, usually from a laser. These externally administered photosensitisers tend to accumulate in rapidly growing tissue, blood vessels and the supporting tissue that grows with malignant tumours. Parenteral administration either by injection or by mouth does produce a period of general photosensitivity and accumulation is in stromal supportive tissue rather directly within growing cells.
Cancer
Photodynamic therapy has attracted most interest as a method for the local eradication of cancer. The initial treatment of patients was of large areas of tumour that could not be treated by other means, or had failed conventional therapy following surgery, radiotherapy or chemotherapy. The treatment of advanced cancers is effective in palliation of some of the difficult symptoms associated with blockage of food or air passages.
Recently great interest has been shown in the treatment of biliary and pancreatic cancer. Bile duct or cholangiocarcinoma can be a relatively indolent tumour but treatment with surgery, radiotherapy and chemotherapy is very difficult.
Pilot studies have demonstrated that endoscopic Photofrin-PDT is also effective in the palliative treatment of hilar cholangiocarcinoma. A Phase III trial compared stenting plus PDT (n=20) with stenting alone (n=19) and showed a prolongation of survival by almost a year in stenting plus PDT group. The most recent study of eight patients, who underwent 1-5 treatments, showed that median survival from the date of the first PDT treatment was 276 days, whereas median survival times were 45 and 127 days for bismuth type III and IV tumors treated with stenting alone. The five-year follow-up data of 23 patients showed that median survival after treatment was 11.2 months for MO patients and 9.3 months for all patients. The 1-year, 2-year, 3-year, and 4-year survival rates were estimated to be 47%, 21%, 11%, and 5%, respectively, for MO patients and 39%, 17%, 9%, and 4%, respectively, for all patients. Preliminary results confirm that endoscopic illumination of the biliary tract is safe and effective for inoperable cholangiocarcinoma and can improve cholestasis, performance, and quality of life for an extended period. Since endoscopic PDT appears to be the first approach leading to an improvement in prognosis, it should be offered to patients with inoperable cholangiocarcinoma. Preliminary studies suggest that operative PDT might also improve survival for those patients undergoing surgical resection
Links
Medscape
Journal of Hepatology and Gastroenterology
Journal of Gastroenterology
Pubmed
0 Comments:
Post a Comment